Checklist for Biologics Cleanroom

A cleanroom is a controlled environment that is designed to minimize contamination and maintain a sterile environment. Achieving the perfect cleanroom can be a daunting task, but with a comprehensive biologics design checklist, you can ensure that your cleanroom meets all necessary standards. With this checklist, you can be confident that your cleanroom will meet the highest standards of cleanliness and safety, ensuring the integrity of your biologics products.
1. Understanding the importance of a cleanroom in biologics manufacturing
Contamination can lead to product failures, reduced shelf life, and even pose risks to patient health.
To ensure the highest level of product quality, regulatory compliance, and patient safety, biologics manufacturers must adhere to rigorous industry standards and guidelines when designing and operating cleanrooms. This comprehensive checklist will guide you through the key considerations and requirements for creating the perfect cleanroom for biologics manufacturing.
Whether you are planning to establish a new cleanroom facility or refurbish an existing one, this checklist will provide you with valuable insights and best practices to achieve optimal cleanliness, sterility, and efficiency in your biologics manufacturing operations.

2. Regulatory requirements and industry standards for cleanrooms in biologics manufacturing
As such, it is crucial to understand the specific regulatory requirements and industry standards that govern the design and operation of cleanrooms in this field.
Regulatory bodies such as the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have established guidelines and regulations for cleanroom design to ensure the integrity of biologics manufacturing processes. This includes selecting appropriate HVAC systems, designing effective filtration systems, implementing proper gowning procedures, establishing robust cleaning and disinfection protocols, and implementing continuous monitoring systems to assess air quality and particle counts.
By meticulously following regulatory guidelines and industry standards, you can create a cleanroom environment that meets the stringent requirements of biologics manufacturing.
3. Selecting the appropriate cleanroom classification for your specific needs
Selecting the appropriate cleanroom classification for your specific needs is a critical step in creating the perfect cleanroom environment for biologics. This classification is determined by international standards such as ISO 14644-1.
To determine the appropriate cleanroom classification, you must consider the nature of the biologics being produced and the level of cleanliness required. Compliance with regulatory guidelines, such as Good Manufacturing Practices (GMP) and Good Laboratory Practices (GLP), should also be considered.
By selecting the appropriate cleanroom classification, you lay the foundation for a controlled and sterile environment that is crucial for biologics manufacturing.

4. Factors to consider when designing the layout and airflow of the cleanroom
Designing the layout and airflow of a cleanroom is a critical step in creating a perfect environment for biologics production. It is essential to consult with experts and consider factors such as ISO classifications, GMP guidelines, and other relevant regulations to ensure that the cleanroom meets the necessary requirements for biologics production.
In conclusion, careful consideration of layout and airflow is essential when designing a cleanroom for biologics production. By paying attention to factors such as segregation of functional areas, unidirectional airflow, proper placement of vents and filters, and adherence to industry standards, you can create a cleanroom that provides the ideal environment to produce biologics while ensuring the highest level of product quality and safety.
5. Essential considerations for HVAC systems and filtration in a biologics cleanroom
When designing a cleanroom for biologics, one of the most critical aspects to consider is the HVAC system and filtration. It is essential to regularly monitor and replace these filters to ensure their effectiveness and prevent any contamination risks.
In addition to HEPA filters, it may be necessary to incorporate additional filtration systems, such as activated carbon filters, to remove volatile organic compounds (VOCs) or other specific contaminants that could be present in the biologics production process. The selection of these additional filters should be based on a thorough understanding of the potential sources of contamination and the specific requirements of the biologics being produced.
Proper air distribution is also crucial in a biologics cleanroom.
6. Ensuring proper gowning and personal protective equipment (PPE) protocols
Ensuring proper gowning and personal protective equipment (PPE) protocols is vital when creating the perfect cleanroom environment for biologics manufacturing. Regular inspections and proper maintenance of PPE should be implemented to ensure their effectiveness and prevent any potential breaches in the cleanroom’s integrity.
In addition to the proper selection and usage of gowning and PPE, it is essential to establish clear protocols for gowning area cleanliness.
7. Implementing effective cleaning and disinfection practices for maintaining cleanliness
Implementing effective cleaning and disinfection practices is crucial for maintaining cleanliness in a cleanroom, especially in the biologics industry where strict hygiene standards are essential. This includes regular cleaning schedules, defined cleaning procedures, and the use of appropriate cleaning agents and equipment.
One key aspect of effective cleaning is the use of disinfectants that are specifically formulated for cleanroom environments.

8. Contamination control strategies for preventing microbial and particulate contamination
When it comes to creating the perfect cleanroom for biologics, implementing effective contamination control strategies is crucial. By establishing clear boundaries and implementing physical barriers, the risk of transfer of contaminants between different production areas can be minimized.
Lastly, a comprehensive training program should be in place to educate all personnel about contamination control strategies, proper cleanroom behavior, and the importance of adhering to established protocols. Ongoing training and reinforcement of best practices will help maintain a culture of cleanliness and awareness among the workforce.
By incorporating these contamination control strategies into the design and operation of a cleanroom facility, biologics manufacturers can ensure the production of high-quality products that meet regulatory standards.
9. Monitoring and maintaining environmental conditions within the cleanroom
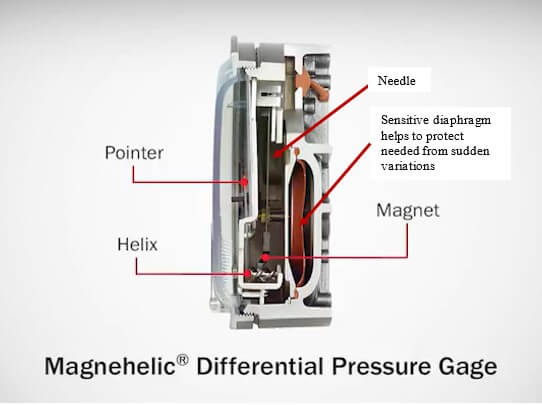
Monitoring and maintaining environmental conditions within the cleanroom is crucial to ensure the integrity and safety of biologics production. Regular checks of pressure differentials using manometers or digital pressure gauges are necessary to confirm that the desired pressure differentials are being maintained.
Regular cleaning and disinfection procedures should be implemented to maintain cleanliness within the cleanroom. This data can be logged and analyzed for trend analysis, allowing for proactive maintenance, and troubleshooting to ensure optimal cleanroom conditions.
By diligently monitoring and maintaining environmental conditions within the cleanroom, biologics manufacturers can ensure the quality, safety, and efficacy of their products.
10. Staff training and ongoing validation of cleanroom processes and procedures
Staff training and ongoing validation of cleanroom processes and procedures is crucial in maintaining the integrity of a biologics cleanroom. If any issues are identified, corrective actions should be implemented promptly to ensure that the cleanroom is restored to its optimal state.
By prioritizing staff training and ongoing validation of cleanroom processes and procedures, biologics manufacturers can create a culture of excellence and ensure the highest level of product quality and safety.
A cleanroom plays a crucial role in ensuring the quality and safety of biologic products. Additionally, a checklist provides a systematic approach to the cleanroom design process, ensuring that no crucial steps are overlooked.
ACH – A Cleanroom Hub is your best choice if your company is undertaking a project for a biologics cleanroom. We specialize in creating innovative cleanrooms that are uniquely suited to meet your demands. Do not wait; contact us immediately. Let us work together to make your vision a carefully thought-out reality.
You can reach out to our experts for more information or request a free estimate.
GET IN TOUCH
Complete the form below to get in touch with our team.
