Cleanroom Pressurization

Cleanroom pressurization refers to the control of air pressure within a cleanroom environment to maintain desired cleanliness levels and prevent contamination.
Cleanrooms have become critical for industries across various industries, including pharmaceuticals, medical, food processing, semiconductors, aerospace, and more. Cleanroom technology ensures high-quality production by keeping environmental parameters like temperature, humidity, and pressure under control while minimizing contaminants in the room.

The increasing demand for medical devices and sterilized pharmaceutical products will only raise the demand for cleanroom technology. According to a report by Transparency Market Research, the cleanroom technology market is currently valued at $4.4 billion, and a 7.6% growth is projected between 2022 and 2032.
Thanks to airflow pressurization, cleanroom technology prevents contaminants from getting into the room. Pressurization in cleanrooms is typically achieved using two main concepts: positive pressure and negative pressure. Let’s explore both of them.
What is Pressurization?
Pressurization is simply the management of airflow within a cleanroom environment to ensure the flow of contamination from the clean room. It is achieved by creating and maintaining the right amount of pressure differentials – either positive or negative.
Ultimately, cleanroom pressurization helps to minimize or eliminate airborne contamination from the surrounding environment or the contaminated cleanroom. When making plans for a cleanroom installation, the intended application will determine whether it features positive or negative air pressure.
What is Positive and Negative Pressure?
A positive-pressure cleanroom has an air pressure level that is greater than the environment surrounding it.
Positive air pressure is created using an HVAC system and HEPA filtration to pump clean, filtered air into the cleanroom. In the event that a window or door is opened or there is a structural breach in the cleanroom, the pressure differential will force the clean air out, preventing contaminated air from seeping in.
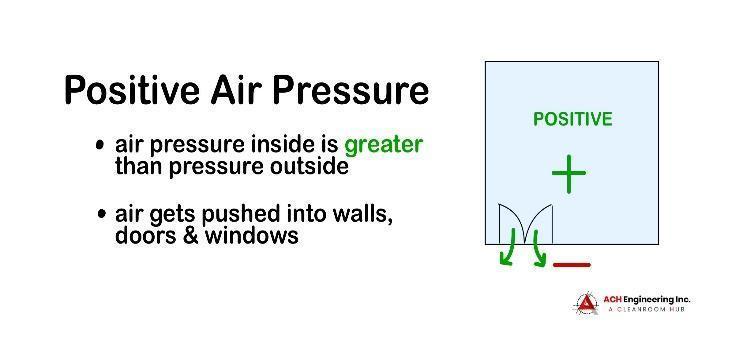
Positive air pressure can be used to protect sensitive products like microchips during the production process or keep vulnerable patients safe from infections and diseases.
A negative-pressure cleanroom, on the other hand, has an air pressure level that is lower than the environment surrounding it.
Negative air pressure is created using an HVAC system to pull more air into the room than the supply air. In the event that the cleanroom is breached, the pressure differential will pull air into the cleanroom, preventing contaminated air from escaping. For optimal functioning of the cleanroom, all doors and windows must be fully sealed, and outtake filters installed to prevent contaminated particles from getting into the room.
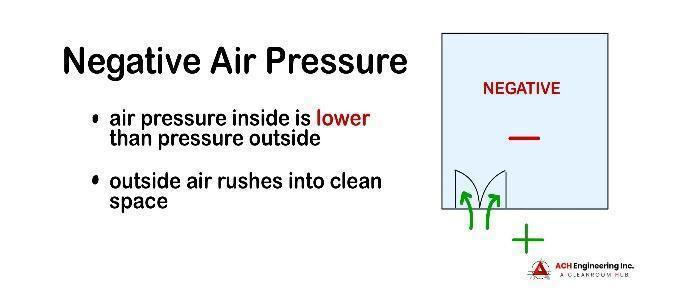
Negative air pressure can benefit clinical or healthcare research facilities; it can protect staff and visitors from potentially contaminated or harmful particles, and improve the quality of work within the facility. Negative air pressure is also useful in pharmaceutical, biochemical and medical industries.
Other Aspects of Cleanroom Pressurization
- Air Supply and Exhaust: Cleanrooms have dedicated air handling systems that supply filtered and conditioned air into the cleanroom space. These systems often consist of high-efficiency particulate air (HEPA) filters that remove airborne particles. The air supply system maintains a higher pressure inside the cleanroom by delivering a higher volume of filtered air compared to the air being exhausted.
- Air Leakage Control: To maintain the desired positive pressure, it is essential to control air leakage points. Common sources of air leakage include doors, windows, vents, and other openings. Seals and gaskets are used to minimize the air leakage and maintain the pressure differential.
- Pressure Monitoring: Cleanrooms employ pressure monitoring systems to ensure that the desired pressure differentials are maintained. These systems use pressure sensors to measure the pressure inside the cleanroom and in adjacent areas. If the pressure drops below the specified level, the monitoring system can trigger alarms or adjust the airflow to correct the pressure imbalance.
The Importance of Pressurization
- Contamination Control: Positive pressurization helps to prevent the entry of contaminants from the surrounding environment. By maintaining a higher pressure inside the cleanroom, the airflow is directed outward, minimizing the ingress of particles and pollutants.
- Product Protection: Cleanroom pressurization ensures the protection of sensitive products, such as electronic components, pharmaceuticals, or precision instruments, from contamination. It helps maintain the required cleanliness levels and quality standards.
- Operator Safety: In certain applications, cleanroom pressurization can also contribute to operator safety. By keeping a positive pressure, potential contaminants, hazardous substances, or pathogens can be prevented from entering the cleanroom and affecting personnel.
Cleanroom pressurization is critical to the success of several industries, including:
- Pharmaceuticals
- Medical cannabis
- Cosmetics
- Hospitals & Healthcare facilities
- Food & Beverage
Manufacturing in the pharmaceutical industry is usually done in controlled environments. As a result, even those who supply packaging materials to pharmaceutical companies must maintain the best possible standards to ensure that the materials are produced within a clean, hygienic, and microbe-free environment.
Growers in the medical cannabis industry understand that consistent crop yield is critical to maintaining profitability. With cleanroom pressurization, medical cannabis cultivators can achieve a well-controlled environment that meets laboratory testing guidelines, prevents the risk of fungal contamination, creates a safer workspace for employees, and ensure consistent crop yields.
Cleanroom pressurization is important in the cosmetic industry, as it helps to control factors such as temperature, humidity, and the movement of airborne particles. Since cosmetic products are applied directly to the human body, ISO 22716 mandates that the bottling or filling of cosmetic products must be done within a cleanroom environment.
In hospitals & healthcare facilities, cleanroom pressurization plays a vital role in ensuring the safety of patients and products. Cleanroom technology is even more important in healthcare facilities today due to the increased rate of hospital-acquired infections. In recent years, though, many healthcare facilities have begun integrating cleanroom technology in isolation areas, quiet rooms, burn units, and even corridors with regular exposure to bio-hazardous particles.
The food & beverage industry is guided by strict manufacturing and packaging procedures to ensure a high level of hygiene and ensure improved product quality. Cleanroom pressurization has a range of benefits for the food & beverage industry, including increased production efficiency, improved shelf life, and maintain food quality in control environment.
Cleanroom pressurization also plays a vital role in the following industries: biotechnology, semiconductors and electronics, E-Liquid vapor, laboratories/R&D Centers, nutraceuticals, and compounding pharmacies.
Avoiding Cross-Contamination
A cleanroom should be first of all clean. This means it is free from contaminants like dust particles, as well as potentially harmful chemical substances. However, humans and other carriers of germs and contaminants can introduce potentially hazardous substances into the cleanroom. Here are five simple ways you can prevent cross-contamination in a cleanroom:
1. Use Cleanroom Garments
Wearing protective garments in the cleanroom will protect you from exposure to potentially harmful substances, and keep the environment safe from contamination. Cleanroom garments include hooded barrier coveralls designed to keep operators safe from hazardous powders, barrier gowns designed for the administration of cytotoxic drugs, as well as those for less critical cleanroom areas like cleanroom smocks and pocket coveralls.
2. Use Protective Footwear
Wearing protective footwear is critical in any cleanroom space. Harmful substances like chemical spills or dust particles may find their way to the floor from time to time, which can lead to injuries and health concerns. Protective footwear and overshoes can be used in the pharmaceutical, food, and healthcare industry to minimize risks from contamination as well as physical accidents like slips and falls.
3. Use Hand Gloves
Hand washing is important to fend off germs and bacteria in any cleanroom and work environment. Our bare hands often have direct contact with dusty surfaces or dangerous particles and can transfer them easily. By wearing cleanroom gloves, you can maintain the sterile condition of the cleanroom and protect yourself from harm.
4. Use Headwear
An uncovered head can easily contaminate a cleanroom environment. Covering the hair, including beards, can protect the cleanroom and work environment from contamination.
5. Use Disinfectants and Cleaning Equipment
Every cleanroom should have a range of cleaning equipment, including mopping systems, chlorine sprays, wall washing systems, detergents, swabs and wipes. Disinfectants are an important way to eliminate viruses and microbes from the cleanroom, and improve the quality of work within the work environment.
Balancing Cleanroom Pressurization
You probably know by now that achieving optimum air pressure level within a cleanroom means balancing pressure differential; it is the maintenance of the quality of supplied air in the room against the air in the surrounding environment. Regulating air pressure differential will help you develop and maintain either positive or negative pressure.

It’s important to note that cleanroom design and pressurization requirements can vary depending on the industry, application, and specific cleanroom classification standards. The specific guidelines and standards of the cleanroom facility should be followed to achieve the desired pressurization and cleanliness levels.
GET IN TOUCH
Complete the form below to get in touch with our team.
