Exploring Cell and Gene Therapy: Cleanroom Requirements and Beyond

Cell and gene therapies are ground-breaking medical innovations with enormous potential for treating a wide range of ailments from the inside out. The accuracy and sterility of these medicines’ manufacturing settings, however, are crucial to their effectiveness. In this blog, we delve into the essential aspects of cleanroom requirements and more within the realm of cell and gene therapy.
Understanding Cell and Gene Therapy
By focusing on the underlying genetic or cellular origins of numerous diseases, cell and gene therapy are cutting-edge medical techniques intended for treating and possibly curing them. In order to restore normal cellular function, correct genetic abnormalities, or improve the body’s inherent ability to combat diseases, these therapies entail manipulating or altering cells and genes within the body of the patient. Both cell and gene therapy are part of the broader field of regenerative medicine.
Crucial Role of the Cleanroom in Cell and Gene Therapy
The production of cell and gene treatments depends heavily on cleanrooms. These carefully regulated conditions are created to reduce the possibility of contamination and preserve particular air quality, temperature, humidity, and other characteristics required for the reliable and secure manufacture of these cutting-edge treatments. For the safety, effectiveness, and quality of the finished products, maintaining a cleanroom environment is crucial due to the sensitivity of cell and gene treatments and the possible risks associated with contamination.

Designing Considerations
Designing a cleanroom for manufacturing cell and gene therapies is a complex process that involves various considerations to ensure the safety, efficacy, and quality of the therapies. Here are some crucial design considerations:
Cleanroom Classification and Environmental Control
- Determine the appropriate cleanroom classification based on the activities being conducted and the level of cleanliness required. This classification is often based on factors like the size and number of airborne particles per cubic meter.
- Maintain strict environmental control over parameters such as air quality (particle count), temperature, humidity, and air pressure differentials to create a consistent and controlled manufacturing environment.
Layout and Zoning
- Divide the cleanroom into distinct zones based on the activities and processes being performed. This helps prevent cross-contamination and allows for the segregation of different manufacturing steps.
- Implement a unidirectional flow of personnel and materials to minimize the risk of contamination.
Gowning and Personnel
- Define gowning procedures and requirements for personnel entering different areas of the cleanroom. This includes proper attire, such as sterile clothing, gloves, masks, and shoe covers.
- Provide designated changing and gowning areas to ensure that personnel follow appropriate protocols before entering the manufacturing space.
HVAC and Filtration
- Install a robust and efficient Heating, Ventilation, and Air Conditioning (HVAC) system with high efficiency particulate air (HEPA) filters to maintain the required air quality and minimize the presence of particles and contaminants.
- Design the HVAC system to create controlled air pressure differentials between different cleanroom zones to prevent the migration of contaminants.
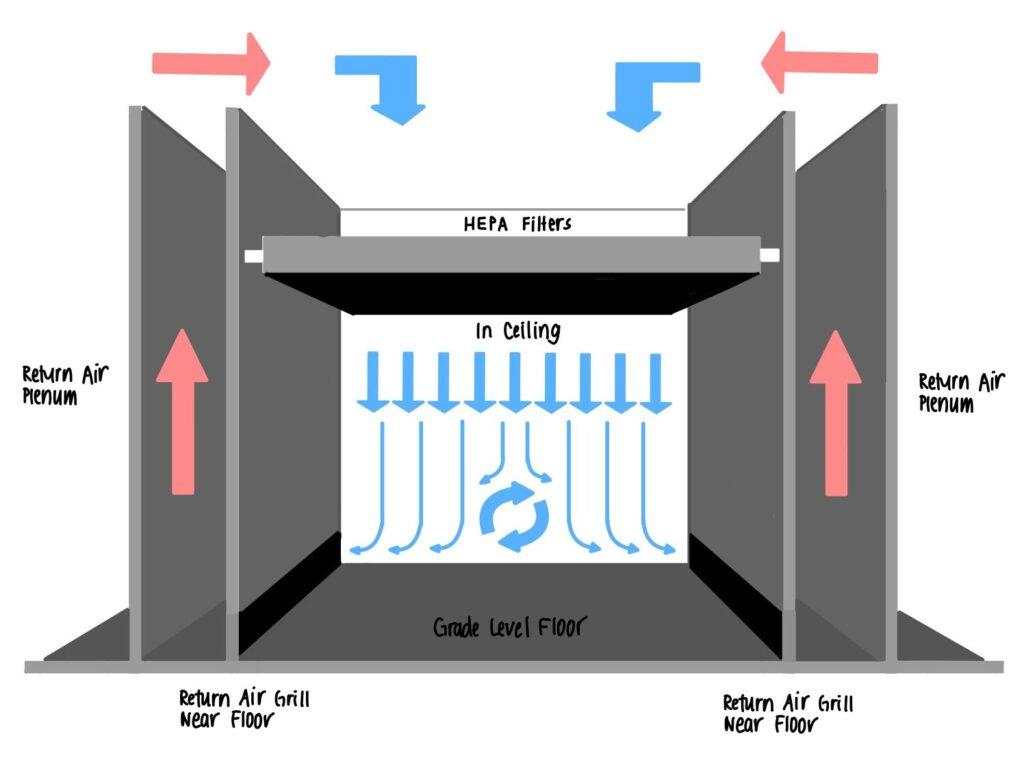
Facility Layout
- Plan the layout to facilitate smooth workflow and minimize the risk of cross-contamination. Separate areas for receiving materials, processing, quality control, and storage should be considered.
- Place equipment strategically to ensure easy access for maintenance and cleaning without disrupting the cleanroom environment.
Surfaces and Finishes
- Use smooth and easily cleanable materials for surfaces, such as walls, floors, and work surfaces, to minimize the potential for particle accumulation and microbial growth.
- Consider antimicrobial coatings or materials that are resistant to chemicals used for cleaning and disinfection.
Aseptic Processing and Containment
- Design specialized areas for aseptic processing, which involve manipulating cells and gene therapies in a sterile environment to prevent contamination.
- Implement containment measures for processes involving potentially hazardous materials or organisms to ensure the safety of personnel and the environment.
Utilities and Services
- Ensure an uninterrupted supply of utilities such as electricity, water, and gases required for manufacturing processes.
- Design systems for waste disposal and handling of biohazardous materials, adhering to safety and regulatory guidelines.

Monitoring and Control Systems
- Implement robust monitoring and control systems to track environmental parameters equipment performance, and personnel activities in real time.
- Install alarms and alerts to promptly address any deviations from specified conditions.
Regulatory Compliance
- Design the cleanroom facility to comply with relevant regulatory standards and guidelines for cell and gene therapy manufacturing, such as those set by the FDA, EMA, and other health authorities.
Validation and Qualification
- Plan for validation and qualification processes to ensure that the cleanroom meets the required standards and performs as intended.
Future Expansion
- Consider future scalability and expansion needs to accommodate increased production capacity or changing manufacturing requirements.
As cell and gene therapy continue to revolutionize medical treatments, the significance of cleanrooms in their production cannot be overstated. By comprehending the unique cleanroom requirements, adhering to guidelines, and emphasizing maintenance, the field can ensure the consistent production of safe and effective therapies.
ARE YOU LOOKING FORWARD TO ESTABLISHING A CLEANROOM FOR YOUR CELL & GENE PLANT? CONTACT US!
ACH – A Cleanroom Hub is the perfect choice for you if your company is starting a cleanroom project for cell and gene manufacturing facility. Our specialty is designing and constructing state-of-the-art cleanrooms that are specifically tailored to meet your needs. Don’t wait; get in touch with us right away, and together, let’s turn your idea into a flawlessly accomplished reality!
Request a free estimate at any time or get in touch with experts to learn more.
GET IN TOUCH
Complete the form below to get in touch with our team.

