NAPRA Compounding Standards: Canadian Pharmacy Compounding

What is NAPRA?
In Canada, pharmacies are required to comply with the National Association of Pharmacy Regulatory Authorities (NAPRA) standards for high-quality sterile compounding pharmacy, ensuring preparation quality and safety. Canadian compounding pharmacy facilities must meet certain requirements for their facilities, equipment, employees, and processes in order to be in accordance with NAPRA guidelines. These prerequisites are as follows:
- Facilities: The compound pharmacies must be constructed to minimize contamination risk by incorporating suitable barriers, air handling units, and cleaning guidelines.
- Equipment: Compounding equipment must be properly maintained and calibrated on a regular basis, and it must meet required accuracy and precision criteria.
- Personnel: To retain their abilities, the compounding staff must be suitably trained and qualified, with continual continuing education and training.
- Processes: Compounding processes must be well-documented, with standard operating procedures (SOPs) in place to guide the compounding process from beginning to end. Quality control processes must also be in place to ensure that the final product is of high quality.
NAPRA Compliance for Pharmacies
Evolving practice and an increased awareness of the inherent dangers of sterile compound preparations to the health of both, patients and the compounding personnel led to the need to review the “Guidelines to Pharmacy Compounding,” published by the National Association of Pharmacy Regulatory Authorities (NAPRA) in October 2006.
The new NAPRA Model Standards for Pharmacy Compounding of Non-Hazardous Sterile Preparations have been adapted from standards originally developed by the Ordre des pharmaciens du Quebec (College of Pharmacists of Quebec), which are in turn based on General Chapter <797> of the United States Pharmacopeia – National Formulary (USP–NF) in effect in the United States since 2004. The purpose of the chapter is to ensure patient safety in healthcare facilities by providing minimum standards for the preparation, storage, and handling of sterile products.
These standards support NAPRA’s Model Standards of Practice for Canada Pharmacists and Pharmacy Technicians, as well as other policies and guidelines that may be in place in provincial/territorial jurisdictions sterile compounding that are compounded in healthcare facilities, including hospitals, pharmacies, and other healthcare settings.
Compounded sterile preparations include the following types of medications:
- Nasal inhalation solutions
- Respiratory therapy solutions
- Solutions for live organ and tissue or graft baths
- Injections (e.g., intramuscular, intravenous, intrathecal, intradermal, subcutaneous)
- Irrigation solutions for wounds and body cavities (e.g., thoracic, spinal, abdominal, pelvic)
- Ophthalmic drops and ointments
- Otic drops for intratympanic administration
- Parenteral nutrition
- Dialysis solutions
- Allergen extracts
- Topical preparations (where sterility is essential to the therapy, e.g., for patients with burns)
- Radio-pharmaceuticals
Pursuant to these Model Standards, sterility is also required for the reconstitution and certain manipulations (according to manufacturers’ instructions) of sterile products approved by Health Canada and for the repackaging of approved sterile products, regardless of the route of administration.
ACH Engineering can help pharmacy clean room design and build their compounding operations in compliance with NAPRA standards.
A. Sterile Compounding (SHD & SNHD):
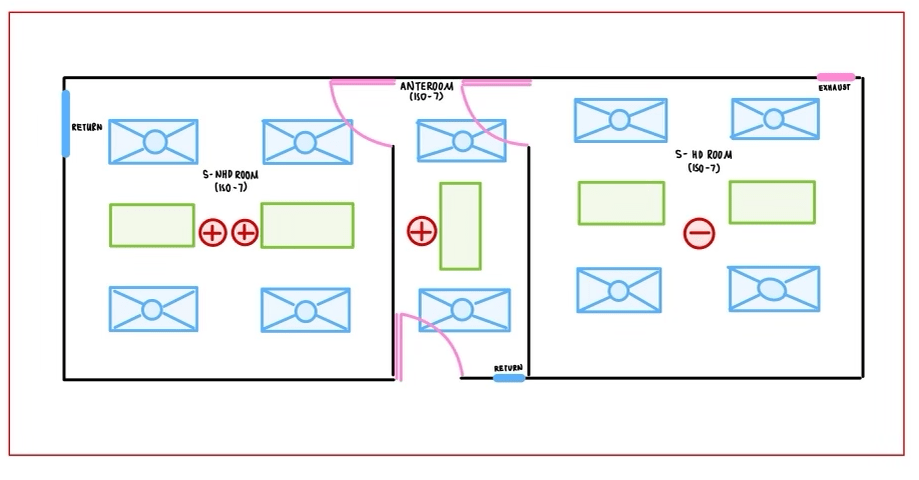
- Provided layout is one of the most common for compounding pharmacies who do both HD and non-HD compounding.
- All rooms need to be ISO 7 in this scenario (SHD, SNHD & Anteroom).
- NAPRA, USP 797, 800 require minimum 30 ACPH
- SHD & SNHD can share anteroom with proper demarcation on floor. This is common to consider the part where door is located as dirty and the other side as clean. To that end, location of HEPA filter and the exhaust are important to assure the right airflow direction. The exhaust will be placed on dirty side near door at floor level when the HEPA filter will be placed on clean side near doors.
- Relative pressures must be as: SNHD positive and highest, Anteroom positive lower than SNHD higher than pharmacy, SHD negative relative to pharmacy. (0.02 WC (5 pa) differential pressure is recommended between ante room & cleanrooms)
- The use of PEC and C-PEC kept within both compounding rooms (Not anteroom). Part of the air change is allowed to be provided by PEC and C-PEC by separate HEPA filters.
- Dedicated AHU can be provided to supply required air for these 3 rooms.
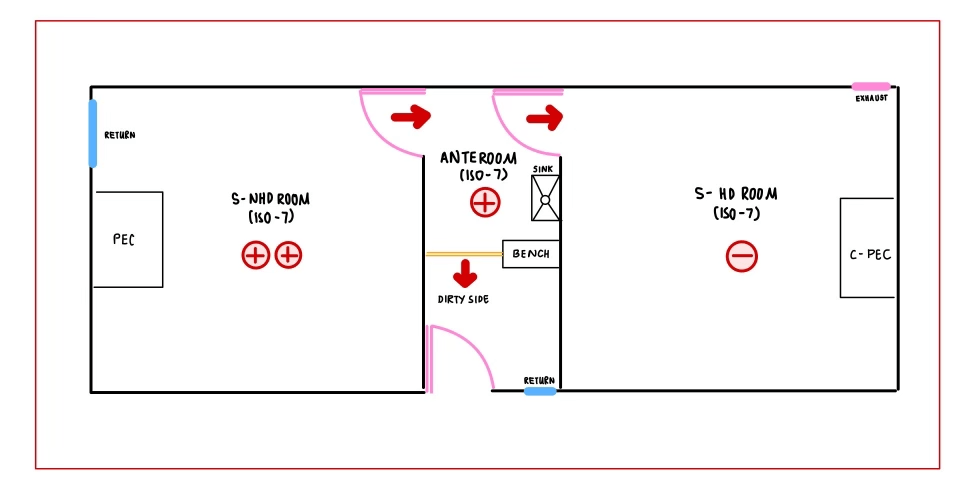
- Supply of air will be thru 99.9% HEPA filter in the cleanroom
- Compounding rooms temperature is required to be 20°C or below all time.
- Number and location of exhausts should consider with the placement of PEC/C-PEC, Tables, Storage racks, Fridges, etc.
- There will be an exhaust up to the roof in the HD & adjacent anteroom.
- Exhaust for HD and NHD rooms have different purposes. HD room should be exhausted outside the pharmacy with roof level fan; at least above 10 ft from the roof and 30 feet away from air intake to AHU unit while NHD room exhaust air can be returned to AHU for recirculation.
- A sink on the clean side of anteroom is needed for employee to clean hand before entering to the cleanroom.
B. Non-sterile HD Compounding (NSHD):
- This is not a classified space hence no HEPA filter is required. However minimum 12 ACPH and the negative pressure is needed so normal diffuser will be provided.
- For same reason no airlock or anteroom is needed for NSHD compounding room. The compounding room is to provide the monitoring system which can be installed for notification purpose for any unusual situation out of the set points (Temp., Pressure, Humidity, air flow).
- NSHD room must be equipped with C-PEC (exhausted outside or with redundant HEPA on floor level).
- Room must be exhausted outside the pharmacy with roof level fan; at least above 10 ft from the roof and 30 feet away from air intake to AHU unit.
- This type of room is addressed in NAPRA under level C requirement.
- An eyewash station and a sink with hot and cold water should be available. A minimum distance of one meter between C-PEC and sink is needed (in case of level C and B the sink can be inside the room).
C. Non-sterile Non-HD Compounding (NSNHD):
- Facility requirements of non-sterile non-HD compounding is addressed in USP 795. USP 795 limits the requirement to “adequate space that is specifically designed for compounding of prescription”. Other addressed requirements are: well lighted, HVAC, orderly placement of equipment and material, availability of potable water.
- NAPRA Model Standard addresses this under the level A, with “separate space/area designated for compounding” and Level B with “separate room that is ventilated” or “has C- PEC”.
- Level A is limited to simple and moderate compounds. Level B addresses complex compounds as well.
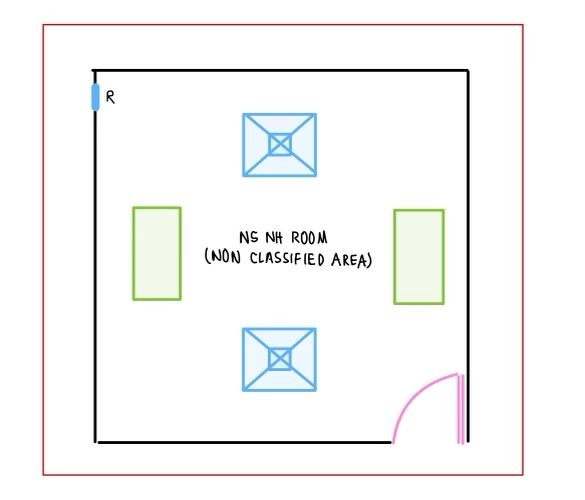
- Since ISO clarification is not needed for this space, it is designed to supply air via normal diffusers. HEPA filter is not required.
- There is no pressure requirement, hence the room can be neutral. This is important as the doors for such rooms may not be kept close at all times.
Overall, NAPRA compliance is critical to ensuring that pharmacy compounding is conducted safely and effectively in Canada, with the goal of producing high-quality, safe, and effective compounded medications for patients.
FAQ:
1. What temperature should a sterile compounding room be?
The room needs to stay cool—at or below 20°C (about 68°F)—all the time. This helps keep the medicine stable and safe to use.
2. Can regular air conditioners be used in compounding rooms?
No. These rooms need special air systems that control particles, temperature, and pressure.
3. What is the difference between Non-Sterile and Sterile Compounding?
- Non-Sterile Compounding refers to medications that don’t need to be sterile, like creams or tablets, but still require certain cleanliness and equipment standards.
- Sterile Compounding involves preparations that must remain free of any microorganisms, like injections or IV solutions. These require strict air handling, temperature controls, and cleanroom conditions.
4. Can one room be used for both sterile and non-sterile compounding?
No. They must be done in separate rooms to avoid mixing and contamination.
5. What are the airlock and pressure requirement for sterile compounding rooms?
The requirements are:
- Cleanrooms and Airlock (anterooms) need to have higher air pressure to keep contaminated air out.
- Hazardous drug rooms need lower pressure, so harmful particles don’t escape.
- The air from hazardous rooms should be pushed outside the building to keep the pharmacy safe.
6. Do pharmacies need to regularly check their compounding rooms?
Yes, they do. Pharmacies should regularly check their compounding rooms to make sure everything is clean, the air pressure is right, and the equipment and vents are working properly. This helps them stay safe and meet NAPRA rules.
7. Do hazardous drug compounding rooms need special ventilation?
Yes, they do. These rooms need to pull air in (called negative pressure) so harmful drug particles don’t escape. The air must be safely pushed outside the building through a fan on the roof. Special equipment called a C-PEC (Containment Primary Engineering Controls) is also used to keep workers safe while handling the drugs.
GET IN TOUCH
Complete the form below to get in touch with our team.
